The COVID-19 Vaccine Program Violates the Nuremberg Code and the U.S. Informed Consent Laws
The COVID-19 Vaccines Are NOT Approved by the FDA
The COVID-19 vaccines are not approved by the FDA as being either safe or effective.
The FDA published a fact sheet prepared by Pfizer-BioNtech that states the following:
Pfizer-BioNtech is only guessing that it “may” prevent COVID-19. That is their hope. But they explain that there is no approved vaccine that will actually prevent COVID-19 and they are not claiming that their vaccine has been proven effective.
The Moderna EUA has the identical song and dance language as is found in the Pfizer-BioNtech EUA. And Moderna makes virtually identical announcements in their fact sheet.
COVID-19 Vaccines are Experimental and Still Under Study
The COVID-19 vaccines are being administered to the public on what is known as an Emergency Use Authorization (EUA) by the FDA.
The COVID-19 vaccines have not been demonstrated to be safe or effective by the FDA. They authorized for use and are being monitored for safety and effectiveness. The safety studies are continuing during the EUA period. The COVID-19 vaccines are experimental vaccines. The COVID-19 vaccine trials are being opened up to include children as young as 6 months. All who take part in the COVID-19 vaccination program are de facto participants in a medical experiment that is being monitored for safety and effectiveness. But they are not being told this.
The COVID-19 Vaccine Program Violates the Nuremberg Code
Dr. Vernon Coleman correctly identifies the COVID-19 vaccine program as a violation of the Nuremberg Code. That is because the COVID-19 vaccines are experimental vaccines that are “authorized,” but they are NOT “approved” by the FDA. They have not been demonstrated to be safe or effective. They have been allowed because the FDA has opined that the potential benefits outweigh the risks. That benefit vs. risk analysis is very low. It allows for a vaccine to be used before the trials for safety and effectiveness are completed. The safety and effectiveness are being monitored. That means that all who get a COVID-19 vaccine are taking part in an experiment to determine the safety and effectiveness of the COVID-19 vaccines. They are unwitting test subjects.
It is unconscionable to trick people into receiving an experimental vaccine. To not inform a person that he is taking part in an experiment violates the Nuremberg Code, which requires that “the voluntary consent of the human subject is absolutely essential” before they take part in a medical experiment.
The Nuremberg Code requires that the consent be knowing consent. The person must be informed that he is taking part in an experiment involving the COVID-19 vaccine that has not been demonstrated to be safe or effective. But the test subjects are not being told this. Indeed, they should be told that the vaccine manufacturers are so unsure of the safety and effectiveness of the vaccine, the test subjects will be monitored to determine if they suffer any dangerous side-effects from the experimental vaccine. But nobody is being told this.
Fraudulent Fact Check by Reuters News Service
Reuters agrees that “[t]he 1947 Nuremberg code does require that human participants in experiments give informed consent.” But Reuters then contests the claim made by Dr. Vernon Coleman and claims that there is no violation of the Nuremberg Code in the administration of the COVID-19 vaccines by falsely claiming that the COVID-19 vaccines are approved. Reuters claims that “it is standard practice for safety monitoring to continue after a vaccine has been approved and rolled-out.” (emphasis added)
That is false. The COVID-19 vaccines have NOT been approved. Reuters is lying to the public. The COVID-19 vaccines are still being tested for safety and effectiveness. For example, the test trials for the Pfizer-BioNtech COVID-19 vaccine are not expected to be completed until January 21, 2023. Indeed, Reuters acknowledges that fact.
The Reuters fact check misleads the public by falsely mixing two concepts and makes a statement that is nonsense. Reuters states that “Pfizer-BioNtech was granted approval for emergency use by U.S. regulators in December 2020.” That is a false statement. The FDA “authorized” Pfizer-BioNtech to administer their COVID-19 vaccine to the public under an Emergency Use Authorization (EUA). An EUA is “permission” by the FDA to administer an experimental vaccine, it is not FDA “approval” of a vaccine that has been demonstrated to be safe and effective.
There is no such thing as approval under an EUA. Indeed, all vaccines used under an EUA are, by definition, unapproved. FDA approval and EUA are mutually exclusive. Reuters deceptively mixed the two concepts to give the false impression that the COVID-19 vaccines have been approved as safe and effective by the FDA, when they have not.
Reuters correctly points out that there is no U.S. Federal requirement for informed consent prior to vaccination. But that is in reference to FDA “approved” vaccines. It is irrelevant regarding experimental “unapproved” vaccines like the COVID-19 vaccines. As I will explain below, there are federal regulations that require informed consent for experimental vaccine programs. Incidentally, many states in the U.S. have informed consent requirements even for FDA approved vaccines.
Federal Informed Consent Requirement
The gravamen of the Nuremberg Code has been adopted as the law in the United States under 45 C.F.R. § 46.111, et seq. and 21 C.F.R. § 50.20, et seq. Section 50.20 states:
Except as provided in §§ 50.23 and 50.24, no investigator may involve a human being as a subject in research covered by these regulations unless the investigator has obtained the legally effective informed consent of the subject or the subject’s legally authorized representative. An investigator shall seek such consent only under circumstances that provide the prospective subject or the representative sufficient opportunity to consider whether or not to participate and that minimize the possibility of coercion or undue influence. The information that is given to the subject or the representative shall be in language understandable to the subject or the representative. No informed consent, whether oral or written, may include any exculpatory language through which the subject or the representative is made to waive or appear to waive any of the subject’s legal rights, or releases or appears to release the investigator, the sponsor, the institution, or its agents from liability for negligence.
21 C.F.R. § 50.23 provides that the only time informed consent does not need to be obtained is when it is not feasible and that determination of infeasibility is documented and certified in writing by the investigator and the physician. Certifiable justification requires ALL of the following:
(1) The human subject is confronted by a life-threatening situation necessitating the use of the test article.
(2) Informed consent cannot be obtained from the subject because of an inability to communicate with, or obtain legally effective consent from, the subject.
(3) Time is not sufficient to obtain consent from the subject’s legal representative.
(4) There is available no alternative method of approved or generally recognized therapy that provides an equal or greater likelihood of saving the life of the subject.
21 C.F.R. § 50.24 provides further justification to waive the informed consent requirement. But that section requires, in pertinent part, documentation of EACH of the following with the concurrence of an independent licensed physician who is not participating in the clinical investigation:
The human subjects are in a life-threatening situation.
Obtaining informed consent is not feasible.
Participation in the research holds out the prospect of direct benefit to the subjects.
The clinical investigation could not practicably be carried out without the waiver.
21 C.F.R. § 50.25 requires that the consent be informed. That means that the person taking part in the medical trial must be told the following pertinent information:
An “identification of any procedures which are experimental.“
“A description of any reasonably foreseeable risks or discomforts to the subject.”
“A description of any benefits to the subject or to others which may reasonably be expected from the research.”
A disclosure of appropriate alternative procedures or courses of treatment, if any, that might be advantageous to the subject.
A statement describing the “confidentiality of records identifying the subject.”
“For research involving more than minimal risk, an explanation as to whether any compensation and an explanation as to whether any medical treatments are available if injury occurs and, if so, what they consist of, or where further information may be obtained.”
“An explanation of whom to contact for answers to pertinent questions about the research and research subjects’ rights, and whom to contact in the event of a research-related injury to the subject.“
“A statement that participation is voluntary, that refusal to participate will involve no penalty or loss of benefits to which the subject is otherwise entitled, and that the subject may discontinue participation at any time without penalty or loss of benefits to which the subject is otherwise entitled.”
Those regulations govern the conduct of the FDA and the vaccine makers regulated by the FDA who conduct medical trials and studies. According to 45 C.F.R. § 46.101, the requirement for informed consent “applies to all research involving human subjects conducted, supported, or otherwise subject to regulation by any Federal department or agency that takes appropriate administrative action to make the policy applicable to such research.”
45 C.F.R. § 46.111(a)(4) requires in pertinent part the following:
Informed consent will be sought from each prospective subject or the subject’s legally authorized representative, in accordance with, and to the extent required by, §46.116.
45 C.F.R. § 46.116(a)(1) explicitly states:
Before involving a human subject in research covered by this policy, an investigator shall obtain the legally effective informed consent of the subject or the subject’s legally authorized representative.
In order for the consent to be informed the test subject, among other things, must be told prior to being administered an experimental vaccine about “any reasonably foreseeable risks … any benefits to the subject … disclosure of appropriate alternative procedures or courses of treatment … an explanation as to whether any compensation and an explanation as to whether any medical treatments are available if injury occurs … whom to contact in the event of a research-related injury to the subject … refusal to participate will involve no penalty or loss of benefits to which the subject is otherwise entitled.”
45 C.F.R. § 46.111(a)(5) requires that the informed consent must be documented in writing. The written documentation requirement can be waived but there is no allowance for a waiver of the informed consent requirement.
Informed consent will be appropriately documented or appropriately waived in accordance with §46.117.
FDA Claims that EUA Medical Countermeasure Products are Exempt from Informed Consent Requirements
The FDA has taken the position that Congress has exempted authorization of vaccines under an EUA from the informed consent requirements of the Nuremberg Code and the above listed federal regulations. 21 U.S. Code § 360bbb–3 provides, in pertinent part, that when an unapproved medical product is authorized for emergency use, the product may be administered provided that the following conditions are met:
(ii) Appropriate conditions designed to ensure that individuals to whom the product is administered are informed—
(I) that the Secretary has authorized the emergency use of the product;
(II) of the significant known and potential benefits and risks of such use, and of the extent to which such benefits and risks are unknown; and
(III) of the option to accept or refuse administration of the product, of the consequences, if any, of refusing administration of the product, and of the alternatives to the product that are available and of their benefits and risks.
The FDA has taken the official position that compliance with the above statutory requirements is sufficient to dispense with the need to obtain informed consent from the EUA vaccine recipient. The FDA alleges:
The FDA further states:
Where did the FDA come up with that? It is required by the EUA statute. While the statute offers a watered-down informed consent requirement; it nonetheless requires informed consent for experimental vaccines being administered under an EUA. The FDA acknowledges that it is not exempt from the Federal law that requires a watered-down informed consent for an EUA under 21 U.S. Code § 360bbb–3, which is the law governing the emergency use authorizations of experimental vaccines. That statute requires the following before administration of a vaccine under an EUA:
Appropriate conditions designed to ensure that individuals to whom the product is administered are informed—
(I) that the Secretary has authorized the emergency use of the product;
(II) of the significant known and potential benefits and risks of such use, and of the extent to which such benefits and risks are unknown; and
(III) of the option to accept or refuse administration of the product, of the consequences, if any, of refusing administration of the product, and of the alternatives to the product that are available and of their benefits and risks.
21 U.S. Code § 360bbb–3(e)(1)(A)(i) (emphasis added)
The FDA thinks that providing a fact sheet to the recipients of the COVID-19 vaccines is sufficient. “Therefore, FDA recommends that a request for an EUA include a “Fact Sheet” for recipients that includes essential information about the product.”
Below are the fact sheets provided by each of the vaccine manufacturers.
Moderna COVID-19 Vaccine Fact Sheet
Pfizer-BioNtech COVID-19 Vaccine Fact Sheet
Basically, the patient is given a fact sheet from each manufacture. Notice that dodge in the Moderna COVID-19 vaccine fact sheet. The fact sheet asks the following question: “ARE OTHER CHOICES AVAILABLE FOR PREVENTING COVID-19 BESIDES MODERNA COVID-19 VACCINE?” Notice that the question only goes to prevention. There is no mention of treatment.
This is the Moderna answer to that question: “Currently, there is no FDA-approved alternative vaccine available for prevention of COVID-19. Other vaccines to prevent COVID-19 may be available under Emergency Use Authorization.” Moderna does not address the question about “other choices” for preventing COVID-19. Moderna limits the response only to vaccines. There is no mention of hydroxychloroquine, ivermectin, or other safe and effective treatment and prevention alternatives. The Pfizer-BioNtech fact sheet contains the same misinformation.
The FDA is reading the statutory requirements under 21 U.S. Code § 360bbb–3 for the administration of an unapproved MCM under an EUA as a waiver of the otherwise required informed consent. The FDA’s position is presumably based on the following language in subsesction (k) of 21 U.S. Code § 360bbb–3:
If a product is the subject of an authorization under this section, the use of such product within the scope of the authorization shall not be considered to constitute a clinical investigation for purposes of section 355(i), 360b(j), or 360j(g) of this title or any other provision of this chapter or section 351 of the Public Health Service Act [42 U.S.C. 262].
The regulations found under found at 45 C.F.R. § 46.111, et seq. and 21 C.F.R. § 50.20, et seq regarding informed consent were enacted under the auspices of those statutes.
Federal EUA Statute is Applicable to Private Parties
The provisions in 21 U.S. Code § 360bbb–3 state, in pertinent part:
With respect to the emergency use of an unapproved product, the Secretary, to the extent practicable given the applicable circumstances described in subsection (b)(1), shall, for a person who carries out any activity for which the authorization is issued, establish such conditions on an authorization under this section as the Secretary finds necessary or appropriate to protect the public health, including the following:
***
Appropriate conditions designed to ensure that individuals to whom the product is administered are informed—
(I) that the Secretary has authorized the emergency use of the product;
(II) of the significant known and potential benefits and risks of such use, and of the extent to which such benefits and risks are unknown; and
(III) of the option to accept or refuse administration of the product, of the consequences, if any, of refusing administration of the product, and of the alternatives to the product that are available and of their benefits and risks.
The COVID-19 vaccine is not like other MCMs. A vaccine is always administered to a healthy person. The person is not receiving the experimental COVID-19 vaccine to treat a disease. They are receiving the vaccine in the hope it will prevent a disease. Thus, all who receive an experimental vaccine to prevent disease are by definition taking part in a medical experiment for which they should be given notice so that they can in turn give informed consent.
COVID-19 Vaccines Proven to be Ineffective
On January 30, 2021, Andrew Court, reported for The Daily Mail that “Democrat Rep. Stephen Lynch has tested positive to COVID-19 after receiving both shots of the Pfizer vaccine.”
Please understand the significance of that occurrence because Rep. Lynch is not the only person to have tested positive for COVID-19 after receiving a COVID-19 vaccination. There has been a slew of such reports. It seems to have become the norm. The COVID-19 vaccine is supposed to prevent someone from getting COVID-19. The whole purpose of the COVID-19 vaccine is to prevent the COVID-19 infection. Indeed, that was the primary focus of the study that resulted in the Emergency Use Authorization (EUA) by the FDA for the Moderna and the Pfizer-BioNtech COVID-19 vaccines.
When you read page 28 of the Pfizer-BioNtech publication titled FACT SHEET FOR HEALTHCARE PROVIDERS ADMINISTERING VACCINE, it reveals that the only criterion reported for establishing the effectiveness of the COVID-19 vaccine is the subsequent infection rate in the study groups.
Changing the Standard
But The Daily Mail article announces that the reason that Rep. Lynch was infected with COVID-19 after being vaccinated is that “Pfizer’s vaccine does not necessarily prevent COVID-19 infection, but is said to be 95 percent effective in stopping the serious symptoms that are caused by the coronavirus.”
That is a problem because the FDA has stated that the Pfizer-BioNtech COVID-19 vaccine has been authorized under the EUA in the hope that it will “prevent” COVID-19 and NOT in the hope it will reduce the severity of COVID-19.
The problem with this new criterion for effectiveness is that it was never studied. If it has now been shown that the vaccine is truly ineffective in “preventing” a vaccine recipient from getting COVID-19 it should be announced as ineffective. The vaccine should be taken off the market. Apparently, that will not happen. Instead, there is now being announced a new criterion for effectiveness that was never studied. And that new criterion is lessening of symptoms.
COVID-19 Vaccines Proven Unsafe
The Pfizer-BioNtech COVID-19 vaccine has not been demonstrated to be safe; indeed, it is downright unsafe. Regarding the safety of the Pfizer-BioNtech vaccine, Pfizer-Biotech admits that “[s]erious and unexpected side effects may occur.”
The Moderna COVID-19 vaccine has not been demonstrated to be safe; indeed, it is downright unsafe. The Moderna fact sheet warns that “[s]erious and unexpected side effects may occur.”
Those warnings have turned out to be prophecies.
The Children’s Health Defense Team reports that “As of Jan. Feb. 4, 653 deaths — a subset of 12,697 total adverse events — had been reported to the Centers for Disease Control and Prevention’s (CDC) Vaccine Adverse Event Reporting System (VAERS) following COVID-19 vaccinations. The numbers reflect reports filed between Dec. 14, 2020, and Feb. 4, 2021. VAERS is the primary mechanism for reporting adverse vaccine reactions in the U.S. Reports submitted to VAERS require further investigation before confirmation can be made that the reported adverse event was caused by the vaccine.”
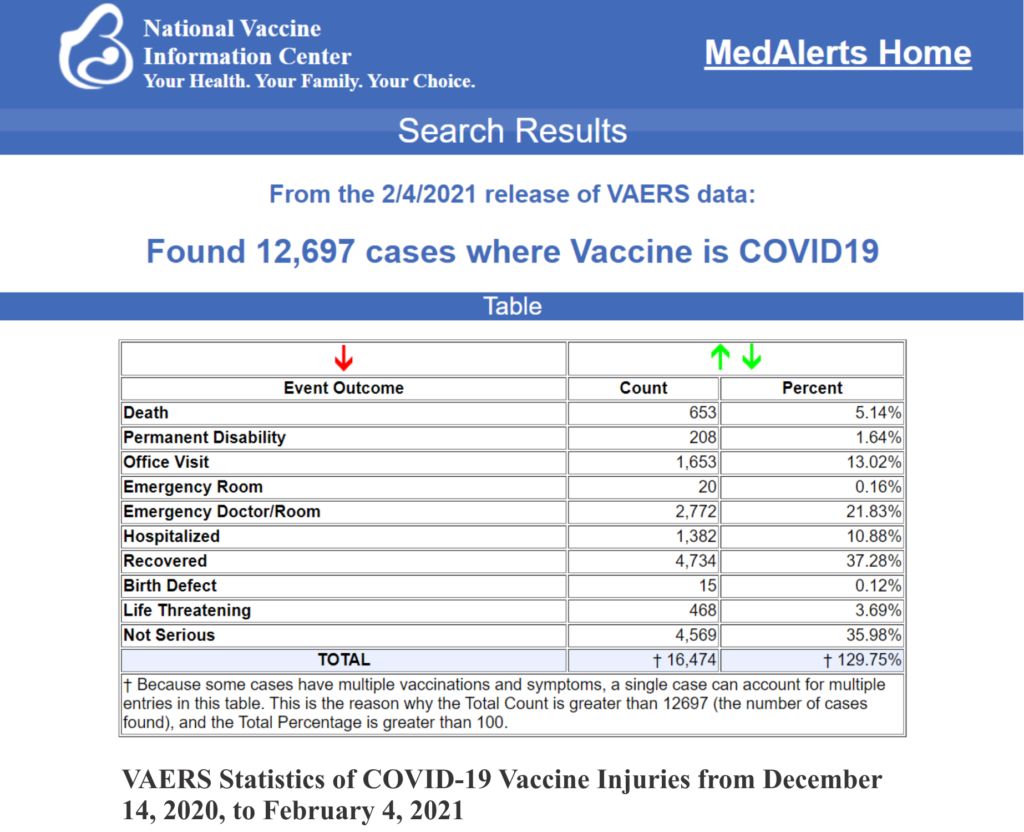
The VAERS database suffers from a systemic flaw that is known to the HHS. That flaw is that the VAERS database underreports the vaccine adverse events by a factor of 100. Indeed, a Harvard study of the VAERS system revealed that “fewer than 1% of vaccine adverse events are reported.” Id. at 6. That means that the approximately 12,697 adverse events reported for the COVID-19 vaccine in the Vaccine Adverse Event Reporting System (VAERS) actually represent 1,269,700 adverse events which include 65,300 deaths from the COVID-19 vaccine.
Vaccine Passports Are on the Way
The L.A. School Superintendent Violates the Nuremberg Code by Proposing that Teachers and Students Be Required to Receive the Experimental COVID-19 Vaccine
The Experimental Vaccines Do NOT Prevent COVID-19 and So They are Changing the Effectiveness Criterion
The FDA and the Vaccine Makers Admit That They Do Not Know If the Experimental Covid-19 Vaccines Are Safe or Effective
The Federal Government Just Agreed to Buy $Billions Worth of COVID-19 Vaccines That Have Not Yet Been Tested for Safety or Effectiveness
Must Watch Debate on the COVID-19 Vaccine: Robert Kennedy vs. Alan Dershowitz
There Is a Cure for COVID-19 But They Are Hiding it From You
Promoting Unsafe and Ineffective COVID-19 Vaccines While Suppressing Safe and Effective Alternative Treatments
The Elderly Receiving the COVID-19 Vaccine are Dropping Like Flies
The Reported 94.5% Efficacy Rate for the Moderna COVID-19 Vaccine is Deceptive
COVID-19 Is No More Deadly Than the Ordinary Flu
The Mainstream Media is Purposely Suppressing the Worst Miscalculation in History
Eminent Doctor Destroys ‘Utterly Unfounded Public Hysteria’ Over COVID-19
Retracted Johns Hopkins Article Proves COVID-19 Death Statistics From the CDC are Manipulated
The President of the AAPS Exposes the Strategem by the CDC and State Health Authorities to Deceptively Inflate COVID-19 Death Statistics
The Rest of the Story Behind the Florida Man Who Died in a Motorcycle Accident and Was Listed as a COVID-19 Death
Washington State Officials Are Again Caught Inflating COVID-19 Death Count
16 thoughts on “The COVID-19 Vaccine Program Violates the Nuremberg Code and the U.S. Informed Consent Laws”
Related posts:
Views: 3
 RSS Feed
RSS Feed
















 February 7th, 2024
February 7th, 2024  Awake Goy
Awake Goy  Posted in
Posted in  Tags:
Tags: 

















Thank you for alerting the world of the gross violation of international laws now taking place by world governments against their populations not seen since the Nazi days.